Generally speaking, surfactants are large hydrocarbon-based molecules that contain a long water-hating/oil loving portion, or “tail,” coupled with a smaller water-loving (hydrophilic) end or “head.”
The water-loving head can carry a neutral, positive, or negative charge. Surfactants with neutral, non-charged heads are called non-ionic. Surfactants with negatively-charged heads are called anionic. Surfactants with a positively-charged heads are called cationic. Cationic surfactants, also known as “quats,” should NEVER be used to clean membrane filtration systems!
The tails are typically comprised of long-chain hydrocarbons and can be linear or branched in configuration. The graphical representation of a surfactant molecule shown below, has a linear tail.
Surfactants do two key things: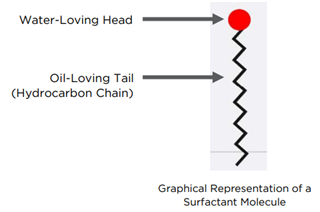
- Lower the surface tension of water, making the cleaning solution less viscous and better able to penetrate surfaces and suspend soils
- Emulsify organic soils, allowing the cleaning solution to carry them away
Two questions to consider:" and then bullet/number the two questions
- Have you ever had a UF or MF membrane unit require an excessive amount of chlorine bleach, or had to perform an additional chlorinated alkaline wash in order to maintain 150 – 180 ppm chlorine on the last wash?
- Have you ever had difficulty controlling micro-counts on a membrane system, even with proper sanitizing frequency and concentration?
If you answered “yes” to one, or both, of the questions above, the issue MIGHT be that the unit is not getting enough surfactant to adequately suspend and remove all the soil residue from the system. Soil left in the system can cause an excessive use of chlorine and can also lead to microbial colonies getting a foothold in the system.
Before exposing the system to excessive amounts of chlorine or sanitizer, try increasing the amount of surfactant added to the early wash(es) of the system.
DO NOT add or increase the amount of surfactant that may be added to the final wash on a system! Adding or increasing the level of surfactant on a final wash could cause rinsing issues and create product quality issues.
Reach out to the RITE Team™ for more information resolving membrane cleaning issues, or making changes to an established cleaning program.
The RITE Team™
By The RITE Team™
Hydrite’s RITE Team™ is a group of experienced professionals tasked to enhance the technical support in the field to introduce innovative solutions that help address critical issues in the food industry.