Chlorine Test Kits
When testing the concentration of sodium hypochlorite, you are performing what is known as an iodometric titration. You are actually titrating iodine species and not hypochlorite.
When testing solutions containing sodium hypochlorite (NaOCl), the general procedure is as follows:
- Obtain a 12 ml sample of solution to be tested.
- Add 10 drops of #5 20% KI (potassium iodide) solution and mix.
- Add 10 drops of #12 42.5% phosphoric acid solution and mix. If chlorine is present, the solution will turn a yellow-amber or amber-brown color.
- Add 2-3 drops of #8 starch solution. The color will change to blue-black.
- While swirling the vial, titrate with #2 0.1N sodium thiosulfate, counting the drops until the solution turns from blue-black to clear.
- Calculation: #of drops x 10 = ppm free chlorine
When the Potassium Iodide (KI) is added to the vial and is then acidified with the phosphoric acid, all of the hypochlorite ion (OCl-) is converted to Tri-Iodide (I3-) according to the equation:
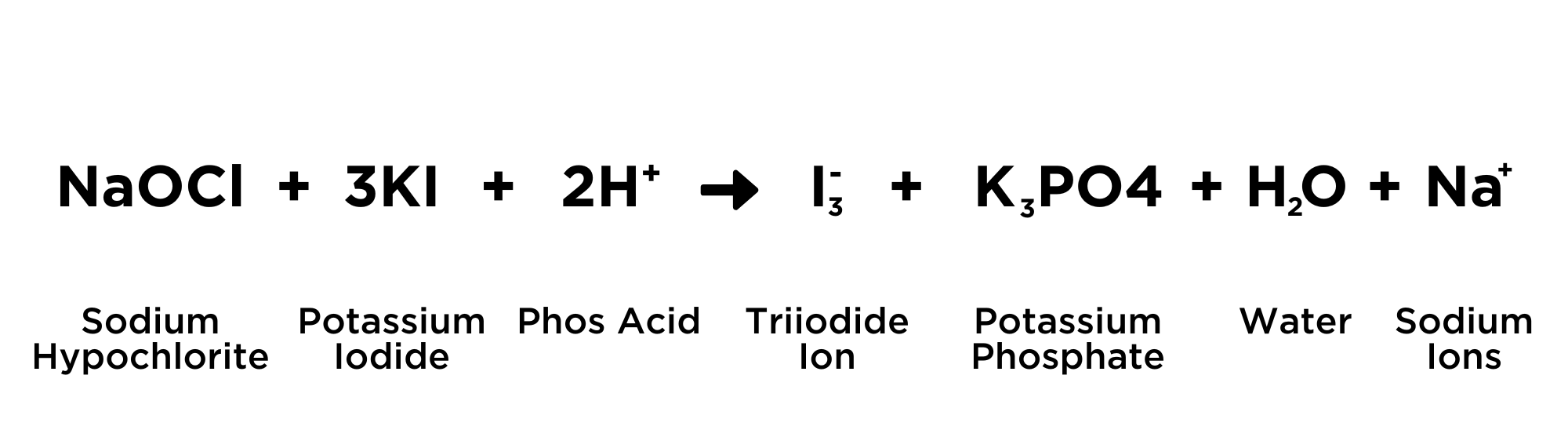
Every hypochlorite ion is converted to an equal number of triiodide Ions. Triiodide ions exhibit an amber-brown color in solution. This is why the solution turns to an amber-brown color if chlorine is present.
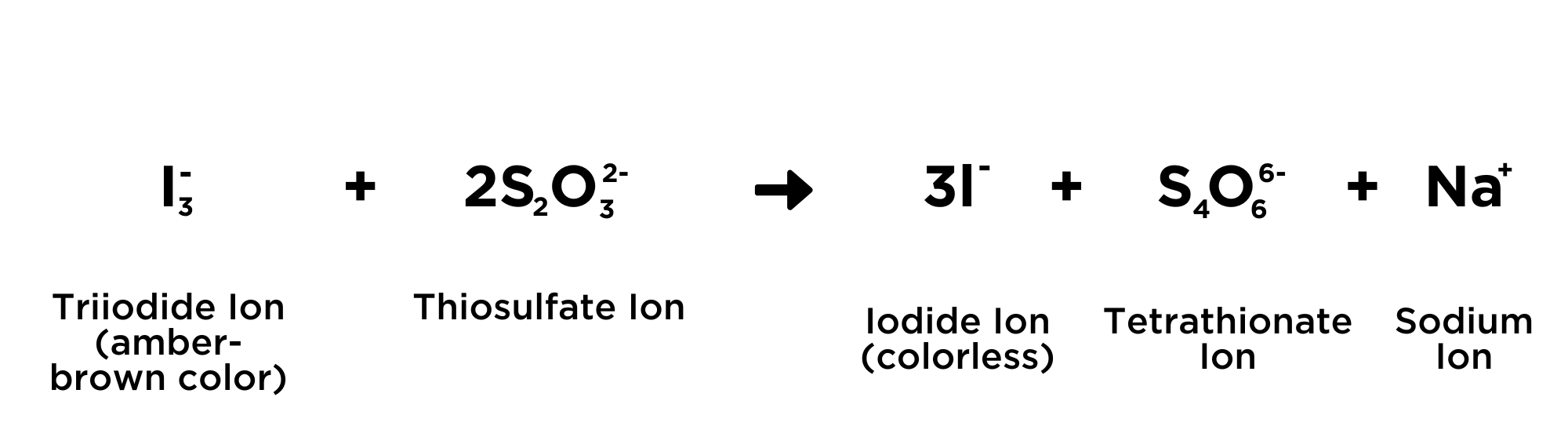
What you are actually measuring/titrating is the Triiodide ions, and not the Hypochlorite (OCl-) ions, according to the equation:
Important Points to Note:
- Similar reactions occur if your sample contains a different oxidizer than hypochlorite—for example, hydrogen peroxide. Therefore, this test kit titrates generic oxidizers, not just chlorine bleach. When the test vial turns to an amber-brown color, it just indicates that your sample contains an oxidizer, it MAY NOT be hypochlorite.
- If you have a test solution that may contain both sodium hypochlorite and hydrogen peroxide, both oxidizers will react with, and neutralize, one another. Only the one present in higher concentrations will partially remain and be detected by the test kit.
Reach out to The RITE Team® for more information on chlorine test kits.

